Recent research from the University of Oregon, backed by the Wu Tsai Human Performance Alliance, aims to do just that.
In a study published in the October 2024 edition of the Bone Journal, researchers in the lab of Nick Willett — an associate professor in the Phil and Penny Knight Campus for Accelerating Scientific Impact and associate director of the Wu Tsai Human Performance Alliance at Oregon — demonstrated that a drug commonly prescribed for its immunosuppressive properties in transplant patients, Tacrolimus (FK506), could also promote bone regeneration for orthopaedic procedures.
Prior to his time at the Knight Campus, Willett was at Emory University, where his lab collaborated closely with the local Veterans Affairs (VA) hospital. There, the team focused on addressing the real-world needs of clinicians. A recurring frustration voiced by healthcare providers was the difficulty patients faced in recovering from spinal fusion surgeries, procedures that connect multiple vertebrae to increase stability or reduce pain.
“I was really inspired by this project, because I liked the idea of talking to the eventual users of this research and getting feedback on what they need and letting that be the inspiration for that work. It really allowed us to see that translation in action,” says Julia Andraca Harrer, a graduate student in the Knight Campus Department of Bioengineering and lead author of the study.
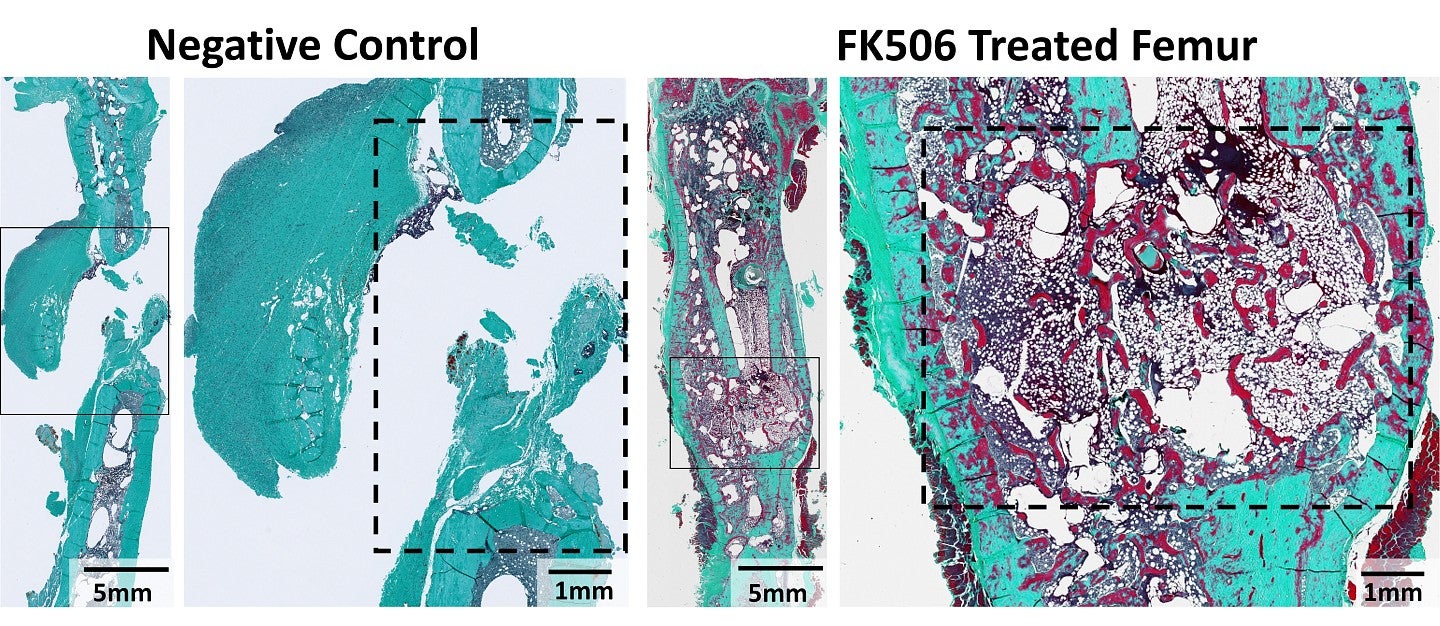
Image: histological staining with Goldner’s Trichrome of negative control and FK506-treated femurs – the boxes denote the defect sites. Image provided by Julia Andraca Harrer.
Motivated by this challenge, Willett’s lab took a novel approach: Could existing drugs be repurposed to fast-track their availability to patients? The team conducted a screen of FDA-approved compounds, and one in particular stood out: Tacrolimus. Known for its role in preventing organ rejection, Tacrolimus also demonstrated the ability to induce bone formation in vitro, a promising first step.
To further investigate Tacrolimus's potential, Andraca Harrer first tested the drug in animal models with bone injuries. She implanted a soft collagen sponge infused with Tacrolimus at the site of a femur injury, then monitored the healing process over several weeks. After 12 weeks, the Tacrolimus-treated femurs in rodents showed significant repair and healed the bone defect injuries, with mechanical properties comparable to those of uninjured bones.
Encouraged by these results, Andraca Harrer then tested Tacrolimus pre-clinical spinal fusion model. In this phase, Tacrolimus-soaked collagen sponges were placed at the surgery site in rabbits, and bone regeneration was assessed six weeks later. As a control, the team also treated some surgery sites with Bone Morphogenetic Protein (BMP), a recombinant protein commonly used for such procedures, but is frequently linked to side effects like excessive bone growth. Remarkably, Tacrolimus not only accelerated the fusion process but also resulted in comparable fusion rates to BMP.
“Commonly used treatments for spinal fusion procedures, like BMP, can have off-target effects and can significantly increase surgical costs, adding to patients’ already high economic and mental burden. This research has the potential to improve healing outcomes for patients and reduce complications caused by current treatments,” says Willett, senior author on the study.
While these findings are promising, much work remains before Tacrolimus can be repurposed for human use in this context. The drug targets a critical pathway in the body, and its off-target effects are not yet fully understood. Future research, including studies in non-human primates, will be essential to determine the optimal dosage, timing, and overall efficacy.
“Going forward, we are excited to continue to push the boundaries of regenerative medicine, seeking new treatments and interventions for patients with bone injuries” says Willett.
-Rachel Bedford, Wu Tsai Human Performance Alliance
