
New research uses the implantable sensors to show how data-enabled resistance training can enhance bone healing
Story by Rachel Bedford
Photos by DustIN Whitaker
December 12, 2024

Tiny implantable sensors are helping University of Oregon researchers optimize the process of recovery from severe bone injuries.
Scientists at the UO’s Phil and Penny Knight Campus for Accelerating Scientific Impact have developed miniature implantable sensors that transmit real-time data about what’s happening at an injury site. In a new study, they use the technology to show that a resistance-training rehabilitation program can significantly improve femur injuries in rats in just eight weeks.
The sensors provide a window into the mechanical properties of the bone, giving scientists detailed ongoing data about the process of healing. If someday applied in humans, these sensors could allow doctors to better tailor a rehabilitation program to an individual patient, monitoring their progress and adjusting the exercises along the way.
The work is a collaboration between the labs of Bob Guldberg, Nick Willett and Keat Ghee Ong in the Knight Campus, and is funded in part by the Wu Tsai Human Performance Alliance. The researchers describe their findings December's issue of Nature Regenerative Medicine.
“Our data support early resistance rehabilitation as a promising treatment to increase bone formation, bone healing strength, and promote full restoration of mechanical properties to pre-injury levels,” said Bob Guldberg, senior author on the paper and vice president and Robert and Leona DeArmond Executive Director of the Knight Campus.
It’s long been understood that post-injury exercise follows a "Goldilocks" principle: Too little or too much can impede recovery, while just the right amount can enhance healing.
Pinpointing the exact type and intensity of exercise needed for the best recovery can be challenging, especially as it varies from patient to patient.
Specialized sensors developed at the Knight Campus could help change that by providing a window into what’s happening inside a healing bone throughout recovery. Originally developed in a collaboration between the Ong and Guldberg labs, these sensors were further improved by recent PhD graduate Kylie Williams.

With the sensors in hand, researchers aimed to test whether resistance running, which is a specific type of recovery exercise, could provide the right mechanical stimulation to improve bone recovery. To do this, they built custom brakes for rodent spinning wheels, which added resistance akin to increasing the incline on a treadmill.
Rats with femur injuries and implanted sensors then ran on either a regular spinning wheel or the modified resistance spinning wheel. The sensors transmitted strain data throughout the exercises, offering researchers a glimpse into the mechanical environment of bone cells during recovery.
Over the eight-week study, researchers monitored the healing process of the injured femurs and found that the resistance-trained rats displayed early signs of bone healing compared to those in sedentary or non-resistance conditions. By the end of the eight-week recovery period, all groups — sedentary, non-resistance, and resistance-trained — showed bone healing.
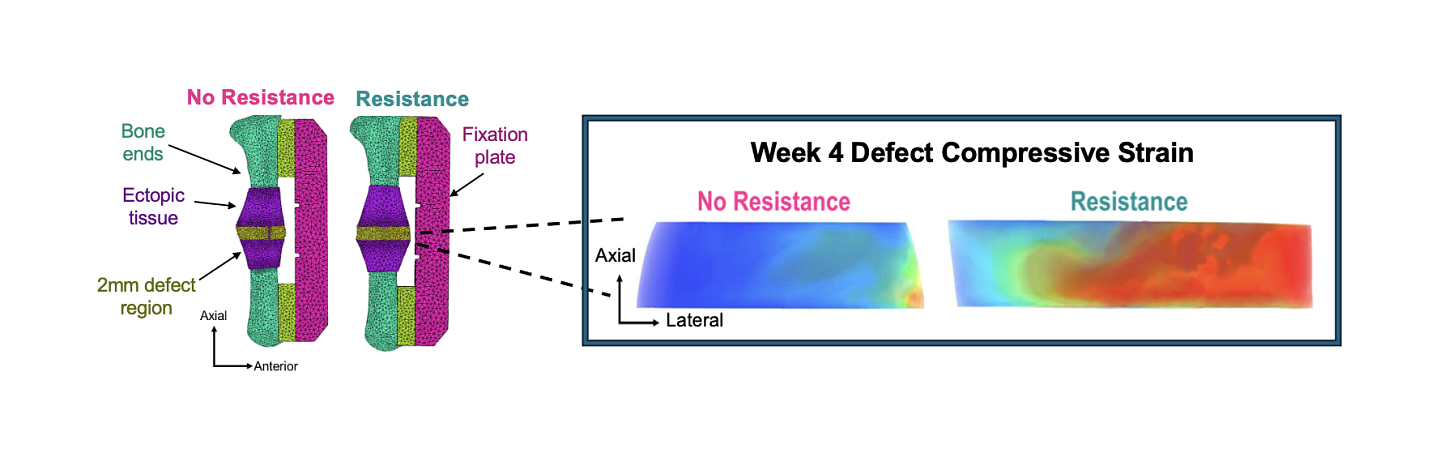
However, the resistance-trained animals had denser tissue, indicating that resistance rehabilitation enhanced bone formation. In fact, the injured bones of the resistance-trained rats exhibited mechanical properties, such as torque and stiffness, comparable to those of uninjured bones.
That indicates resistance training enhances recovery, even without any additional drugs or biological stimulants, Guldberg said.
Biological agents like BMP, a molecule that promotes bone growth, are often used in regeneration studies. However, Guldberg’s team demonstrated complete functional recovery through resistance training alone, underscoring its potential for clinical application.

One of the most impactful aspects of this work is that our resistance rehabilitation could regenerate the femur to normal strength within eight weeks without biological stimulants, and we’re really excited about that,” said Williams, the lead author of the study.
One limitation of the study is that all animals received a constant level of resistance throughout the experiment. However, researchers in the Guldberg lab are now investigating how increasing or decreasing levels of rehabilitation intensity across weeks of healing may affect bone regeneration.
Although the research was conducted in rodents, the team hopes that data-enabled rehabilitation also can be used to improve healing in human patients who sustain musculoskeletal injuries. Toward that goal, Penderia Technologies, a Knight Campus start-up company housed in the Papé Family Innovation Center, is working on further improvements to the implantable sensors, including a battery-free design and wearable monitors to aid use in human patients. After graduation in December, Williams will be joining Ong and the growing Penderia team to further explore the clinical translation of the preclinical strain sensors used in this study.
“We are hopeful this work can one day be translated to clinical settings, where these sensors can capture personalized measurements that account for injury type and severity to best inform rehabilitation decisions,” Guldberg said.
This research was supported by the National Institutes of Health, the VA merit grant and the Wu Tsai Human Performance Alliance Initiative.
The Wu Tsai Human Performance Alliance at Oregon Based in the Knight Campus

