What if we could accelerate healing following serious injuries? Recently published research, supported by the Wu Tsai Human Performance Alliance, focuses on enhancing soft, flexible gels to support regenerating cells and accelerate healing.
Scientists have been studying soft, jelly-like structures known as hydrogels for years, but new research from the lab of Knight Campus professor Marian Hettiaratchi casts them in a new light. Hydrogels can be implanted to mimic tissue and provide structural support to regenerating cells. This research, funded by the Wu Tsai Human Performance Alliance, appeared in the February 2024 Journal of Materials Chemistry B Emerging Investigators 2024 themed collection.
The Hettiaratchi lab specifically focused on building a pipeline to identify the best combinations of hydrogel parameters for potential regenerative uses. With ongoing development, this approach could deliver molecules at precise doses and times to help patients heal faster.

“While this was an initial proof of principle, we are excited about using these approaches to be able to tune the release of molecules that facilitate regeneration in many different tissues,” says Marian Hettiaratchi, who led a group of Bioengineering Graduate Students, Knight Campus undergraduates, and external collaborators at Oregon State University to optimize this technology.
Variations in the physical properties of the hydrogels, such as stiffness and the time it takes the hydrogel to solidify, significantly impact functionality during regeneration. However, testing each physical parameter individually is time and resource-intensive. To tackle this challenge, the Hettiaratchi lab, alongside Kaitlin Fogg, an Assistant Professor at Oregon State University, created a pipeline that included a computational model predicting the optimal hydrogel combinations. Through this approach, researchers determined the desired physical parameters of the hydrogel and then used the computational model to predict the exact recipe combinations to achieve that. This approach significantly enhances hydrogel screening efficiency and enables researchers to customize hydrogels for various clinical needs.
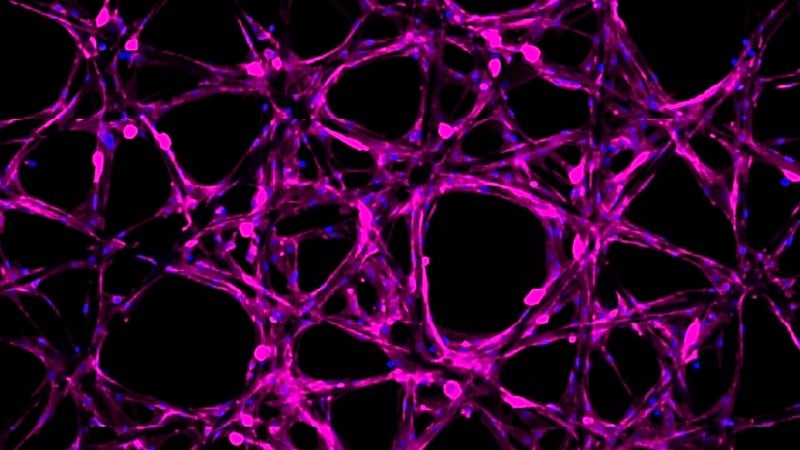
Another remarkable feature of these hydrogels is their capability to embed and release specific molecules. In this study, the Hettiaratchi lab filled the hydrogel with BMP-2, a molecule known for promoting bone growth, and showed that the hydrogel could release the BMP-2 molecules at different rates. Utilizing hydrogels in this manner could enable clinicians to administer precise concentrations of molecules that promote regeneration or medications directly to a wound site.“
These structures and the computational model are so multifunctional and versatile,” said Alyicia Galindo, a Knight Campus bioengineering PhD student and co-lead author of the study. “We can go back to the model to estimate what other parameters need to change to have these hydrogels deliver compounds for different tissues. Here we showed what it may look like in the context of regenerating bone, but we’re already thinking beyond that to more tissues like the spinal cord.”.
Supported by a grant from the National Institutes of Health known as an R21 Trailblazer Award, designed to assist new labs in tool development, the work stands out not only for its scientific progress but also for its collaborative nature. Hettiaratchi collaborated with co-investigator Fogg, an assistant professor at Oregon State University, to build the study’s computational models. Work in the lab with the hydrogels was first started by chemistry PhD student Veronica Spaulding. This study was then spearheaded by Galindo and Knight Campus undergraduate scholar Esther Mozipo.
“The Hettiaratchi lab is a really collaborative environment,” said Jenna Khachatourian, Knight Campus Undergrad scholar and author on the paper. “Everyone has their own level of expertise that they contribute, and it allows room for everyone to grow and learn from one another.”
This pipeline for developing customized healing hydrogels holds significant promise for accelerating the screening of physical parameters for hydrogels and infusing them with substances that promote healing. While the current publication focused on using hydrogels to release molecules that stimulate bone growth, the Hettiaratchi lab envisions extending this approach to various cell types and injuries, including skin wounds and spinal cord injuries. This work also highlights the power of collaboration across students, labs, and the state of Oregon in revolutionizing new approaches to achieve regeneration.
— Rachel Lukowicz-Bedford
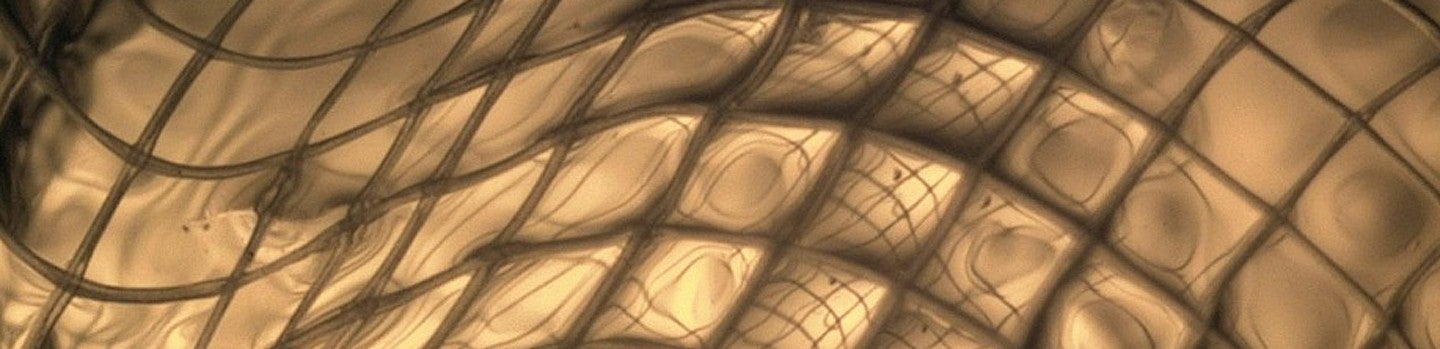
Header Image Caption: Close-up of hydrogel inside polycaprolactone mesh tube

